What Is a Ceramic Crucible?

What Is A Ceramic Crucible?
A crucible is a container designed for extreme temperature applications, particularly in melting metals. Ceramics, with non-reactive surfaces, are crucial for making crucibles. Ceramic crucibles, including quartz, corundum, boron nitride, zirconia, etc., have been used in metalworking since 5000 BC. Their design has evolved with metallurgical advancements. Various raw minerals can be used to create ceramic crucibles, and the choice depends on the intended application's temperature and pressure requirements.
Typical Ceramic Crucibles
Alumina Crucible
Alumina crucibles are a scientific name for corundum crucibles with about 99.7% of the material being alumina Al2O3, having traces of MgO and SiO2. Due to their low cost and ability to resist a range of temperature conditions, alumina crucibles are particularly popular. They are strong and resistant to melting, high temperatures, acid and alkali, rapid cooling, intense heat, and chemical corrosion. Corundum crucible is a great material for melting samples - it works well for some weakly alkaline materials such as anhydrous Na2CO3, but not for Na2O2, NaOH, etc. Fluxes are compounds that are both strongly alkaline and acidic and are used to melt samples. In a redox atmosphere of 1650°C–1700°C, the 99.70% pure alumina crucible has good high-temperature insulation and mechanical strength, and the maximum temperature can quickly approach 1800°C. Depending on the circumstances of the application. Many sizes and shapes of alumina crucibles are available.

PBN Crucible
Common varieties of Boron Nitride (PBN) are Cubic Boron Nitride (CBN) and Hexagonal Boron Nitride. The boron nitride crucible is typically made of P-BN. P-BN ceramics are excellent heat dissipation and high-temperature insulating materials because of their excellent heat resistance, thermal stability, thermal conductivity, and dielectric strength at high temperatures. Due to PBN's excellent chemical stability, it can withstand the erosion of the majority of molten metals. It is ideal for harsh environmental conditions like semiconductor manufacturing processes because of its high thermal conductivity and low thermal expansion properties depending on the substance employed. PBN crucibles are frequently employed in the smelting of semiconductors and metals. With atmospheric protection, it can withstand temperatures of up to 2100°C and 1800°C, respectively. generally protected with argon or nitrogen (atmospheric protection is to prevent the crucible from oxidation).

Further Reading: Use Guide of Pyrolytic Boron Nitride Crucible
Zirconia Toughened Alumina Crucible
Zirconia Toughened Alumina (ZTA) crucibles are advanced ceramic vessels that combine the high-temperature resistance of alumina with the toughness and durability of zirconia. Comprising about 90% alumina (Al2O3) and 10% zirconia (ZrO2), these crucibles offer a unique balance of mechanical strength and thermal stability. ZTA crucibles find applications in various high-temperature processes where the simultaneous requirements of strength and resistance to thermal shock are essential. Their versatility makes them suitable for tasks ranging from the melting of metals to the synthesis of advanced materials.
Magnesium Oxide Crucible
Magnesium Oxide (MgO) crucibles are crafted from magnesia, exhibiting exceptional resistance to chemical corrosion and high-temperature stability. With a composition primarily consisting of magnesium oxide, these crucibles provide a reliable container for the melting and heating of metals. Due to their robust nature, MgO crucibles are commonly used in laboratories and metallurgical processes where a durable vessel is essential.

Beryllium Oxide Crucibles
Beryllium Oxide (BeO) crucibles are advanced ceramic containers known for their unique properties, including high thermal conductivity and resistance to thermal shock. Composed of beryllium oxide, these crucibles are suitable for applications requiring rapid heating and cooling cycles. They find utility in various high-temperature processes, offering a robust solution for tasks demanding precise control over thermal conditions.
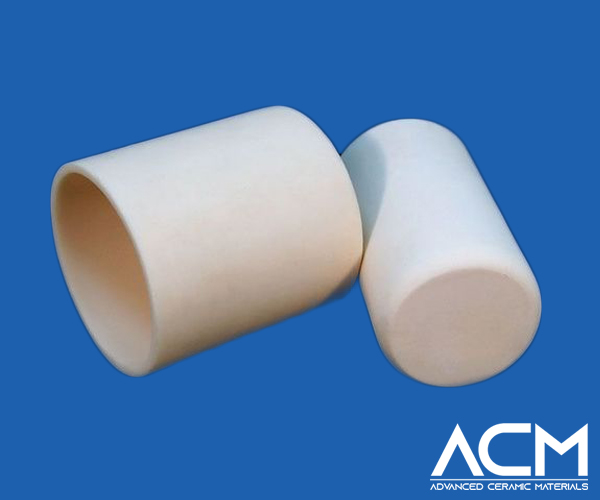
Zirconia Crucible
Zirconia crucibles, made from zirconium oxide, stand out as one of the most refractory materials in nature. With a higher melting point than zirconium, these crucibles successfully smelt precious metals like platinum, palladium, ruthenium, and cesium, along with their alloys. While more expensive than alumina, zirconia is a special oxide refractory material that can withstand high-temperature furnaces above 2000 ℃, making it irreplaceable for certain applications.
Graphite Crucible
A graphite crucible, commonly employed for melting non-ferrous metals like gold, silver, aluminum, or brass, offers high-temperature endurance and low reactivity with molten metals. Comprising carbon material, graphite is often combined with clay, molded, and subjected to high temperatures to create a robust container. While graphite crucibles share some fragility with ceramic vessels, careful handling is essential. The inherent softness of graphite extends its utility to applications such as lubricant formulations, structural materials, and metallic alloys.

Proper storage and conditioning are vital for graphite crucibles. It's essential to precondition a new graphite crucible before use. After two hours at 500 degrees Fahrenheit (260 degrees Celsius) in the oven, the crucible should be slowly cooled in a dry area to prevent splitting due to moisture. Wet crucibles require drying off and reconditioning, and they should never be stored near moisture.
Conclusion
The steady progression of chemical processes depends on ceramic crucibles. Ceramic crucibles are crucial pieces of chemical equipment used for melting down materials, an effective way to reuse scrap items. Ceramic crucibles make it easier to recycle metallic materials because they can easily be cast into new objects or combined into new alloys.
{{item.content}}
LEVE A REPLY
{{item.children[0].content}}
{{item.content}}
LEAVE A REPLY
SUBSCRIBE OUR NEWSLETTER
- How PBN Crucibles Ensure the Quality of GaN & SiC Epitaxial Materials
- SiC vs. Quartz Focus Rings: A Cost and Performance Analysis for Advanced Etch
- AlN Ceramic Substrates: Enabling Next-Gen Electrostatic Chucks
- The Amor of Semiconductor Tools: Why High-Purity Al2O3 & AlN Are Preferred for Plasma Process Chambers
- Silicon Carbide - Ultra-High Temperature Ceramics for Extreme Environments