What is Cubic Zirconia?
Introduction: Why Cubic Zirconia?
Looking for the sparkle of a diamond without the hefty price tag? Cubic zirconia might be the perfect gem for you. Since its introduction in 1976, this lab-created gemstone has become a popular and affordable alternative to diamonds, closely mimicking their brilliance, clarity, and durability.
Manufactured in laboratories, cubic zirconia shares many aesthetic, optical, physical, and chemical characteristics with natural diamonds, making it a significant competitor in the gemstone market. In this guide, we’ll explore what cubic zirconia is, how it’s made, and why it continues to captivate jewelry lovers worldwide.
What is Cubic Zirconia and How is it Made?
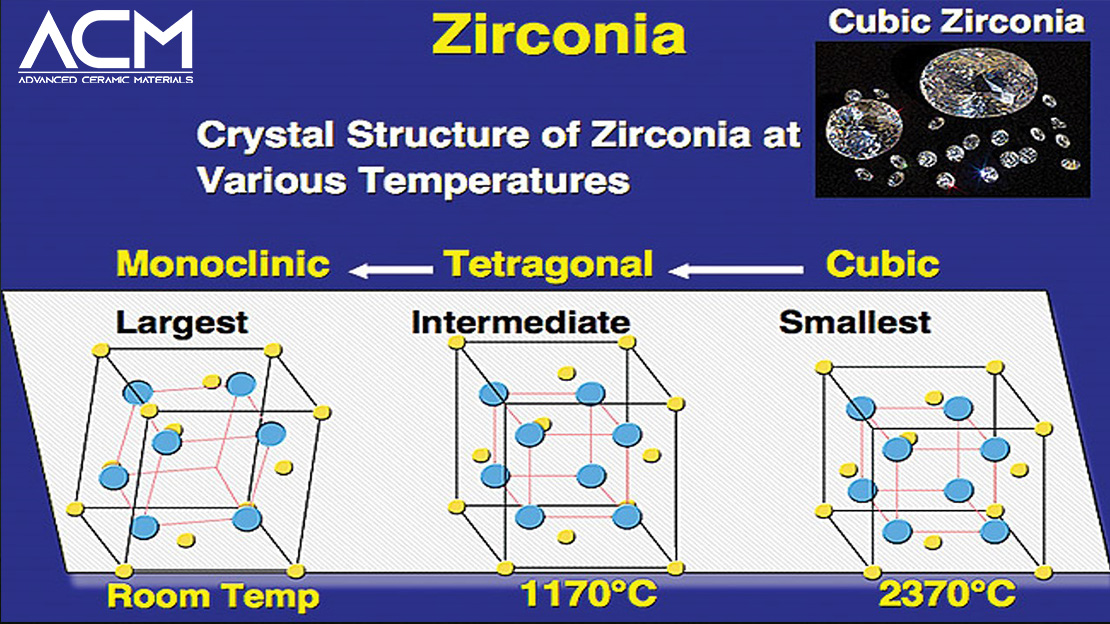
Cubic zirconia is a synthetic gemstone. It is a crystalline cubic form of zirconium dioxide. Although extremely rare, cubic zirconia can be found in nature as tiny crystals within baddeleyite and zircon. All cubic zirconia stones on the market today are man-made, which means they are only made in labs. They are created from high-purity zirconium oxide powders stabilized with magnesium and calcium.
Zirconium oxide is heated in a metal chamber while calcium and magnesium are added to create cubic zirconia. The temperature is then raised to approximately 5,000°F (about 2,760°C). The amount of each element is carefully regulated, and occasionally, additional ingredients are added to create results that resemble genuine diamonds. The product leaves the metal chamber and cubic zirconia crystals start to grow. The stone is then sliced. Cubic Zirconia stones can be shaped in a variety of ways, including emerald, oval, pear, and more cuts.
Cubic Zirconia vs. Diamond: Key Differences
Cubic zirconia and diamonds might have a somewhat similar appearance, but they have vastly different compositions.
Hardness and density vary
The hardest stone known to man is a diamond, but cubic zirconia rates far lower on the hardness scale. This is because diamonds' brilliance and extraordinary hardness are due to the compacted carbon atoms that make up their structure. To determine whether a diamond is indeed a diamond or cubic zirconia, various hardness comparisons can be used. Diamonds can also be tested by weighing the gemstone. Although a diamond and cubic zirconia might have the same physical size, zirconium dioxide has a little higher density than carbon at the molecular level. A cubic zirconia stone will therefore weigh more than a diamond of the same size.
Genuine diamonds are more expensive
The primary reason cubic zirconia diamonds are so popular is that they almost always cost less than diamonds, even though the price of a diamond may change based on its size or other characteristics. A 1-carat cubic zirconia diamond typically costs about 1/100th of what a 1-carat diamond would cost.
The ratio only gets bigger as the carat size gets bigger. The fact that cubic zirconia has little to no resale value in contrast to diamonds, which can often be sold for at least 50% of their original cost, poses a possible problem.
Inclusions and possible flaws
Cubic zirconia is devoid of the inherent flaws present in real diamonds because they are created in a laboratory. This indicates that they have almost perfect clarity. Due to their nearly too-perfect look, they may come off as false. Natural flaws called inclusions can be seen inside a diamond. Even though diamonds without any inclusions are theoretically true, they are incredibly rare and expensive.
Inclusions are minuscule defects that are present in natural diamonds as well as lab-grown diamonds. Despite having no inherent internal faults, cubic zirconia can exhibit clear evidence of its lab-grown origins, such as minute gas bubbles containing the zirconium dioxide powder used to make it.
Appearance
Gemologists are aware that a "white" diamond can contain a yellow, gray, or brown tinge, despite the misconception that a diamond should appear clear throughout. Cubic zirconia is likely to have no color at all.
Light dispersion
When white light enters a diamond, disperses (or divides), and exits the stone at various angles with colored light, this is known as a dispersion in a gemstone. Compared to diamonds, cubic zirconia has a higher degree of dispersion. The dispersion rate ranges from 0.058 to 0.0666. The dispersion rate of diamonds is 0.044. As a result, cubic zirconia will sparkle more brilliantly than diamonds.
Pros and Cons of Cubic Zirconia
Pros
1. Affordable Price
One of the biggest advantages of cubic zirconia is cost. It offers the look of a diamond at a fraction of the price, making it ideal for budget-conscious buyers or large-scale production.
2. Brilliant Sparkle
As mentioned before, CZ has a higher dispersion rate than diamonds (around 0.058 vs. 0.044), which gives it a strong “fire” under light. This makes it visually striking, especially in well-cut stones.
3. Various Shapes and Sizes
Being lab-grown, CZ can be manufactured in a wide range of shapes and dimensions. This allows for more flexibility in design—from fine solitaires to elaborate fashion pieces.
4. Flawless Appearance
Unlike natural gemstones, cubic zirconia is typically free from inclusions and blemishes. This results in a clean, uniform appearance that appeals to customers looking for a flawless finish.
5. Reasonable Durability
With a Mohs hardness of 8–8.5, CZ is hard enough for most everyday jewelry use. While it’s softer than diamonds, proper care and protective settings can extend its life.
Cons
1. Lower Hardness
Compared to diamonds (Mohs 10), CZ is more susceptible to scratches and surface wear over time—especially in rings or bracelets that face frequent contact.
2. Heavier Weight
Cubic zirconia has a higher density (about 5.6–6.0 g/cm³) than diamond (around 3.5 g/cm³), making it noticeably heavier. This difference is subtle but can be identified by trained jewelers or experienced wearers.
3. No Resale Value
CZ is not considered a precious gemstone, and it typically has little to no secondhand market value. It's a good option for style and sparkle, but not as an investment.
4. Artificial Look
Its flawless clarity and intense sparkle can sometimes look "too perfect," especially under bright lighting. For customers seeking the natural imperfections of mined stones, this may be a drawback.
5. Sensitivity to Heat and Chemicals
CZ is more sensitive to high temperatures and strong chemicals than diamonds. It’s best cleaned with mild soap and water to avoid clouding or surface damage.
How to Choose and Care for Cubic Zirconia Jewelry
When shopping for cubic zirconia jewelry, look for stones with high clarity and good cut quality to maximize sparkle. Although CZs are lab-made and typically flawless, some may have tiny gas bubbles. Always buy from reputable sellers who provide certification or guarantees.
To keep your CZ jewelry shining, clean it regularly with mild soap and warm water. Avoid harsh chemicals and ultrasonic cleaners, which can damage the stone or setting. Store your pieces separately to prevent scratches.
Conclusion
Cubic zirconia is often labeled as "fake" or "simulated" diamonds, but in reality, it is a genuine gemstone with impressive durability and a striking resemblance to diamonds. Rather than being mere imitations, cubic zirconia stones stand as a beautiful and affordable alternative for those seeking sparkle without the high price.
Whether you’re shopping for everyday jewelry or a special gift, cubic zirconia offers excellent value and versatility. We hope this guide has helped you better understand the qualities and benefits of cubic zirconia. For more information on ZrO2 ceramic materials and our latest products, please visit our homepage.
Ready to explore the brilliance of cubic zirconia? Discover our collection today and find the perfect sparkling gem for you!
{{item.content}}
LEVE A REPLY
{{item.children[0].content}}
{{item.content}}
LEAVE A REPLY
SUBSCRIBE OUR NEWSLETTER
- AlN Ceramic Substrates: Enabling Next-Gen Electrostatic Chucks
- The Amor of Semiconductor Tools: Why High-Purity Al2O3 & AlN Are Preferred for Plasma Process Chambers
- Silicon Carbide - Ultra-High Temperature Ceramics for Extreme Environments
- Aluminum Oxide Ceramics: Properties and Applications
- Boron Nitride Coatings: The Solution for Molten Metal Applications