Zirconia Ceramic Properties: A Summary
Introduction
Zirconia (ZrO2), also known as zirconium dioxide, is a ceramic material known for its outstanding strength, toughness, and thermal stability. First identified in the 18th century, zirconia has evolved into a key player in high-performance engineering ceramics. Due to its superior mechanical performance and biocompatibility, it is widely used in structural applications, dental implants, oxygen sensors, and cutting tools. This article provides a concise summary of the essential properties of zirconia, emphasizing its utility in challenging industrial and biomedical environments.
Physical Properties of Zirconia
Zirconia is typically white in its pure form, but can appear yellow or gray depending on additives and grain size. It is odorless and exhibits a high density of 5.68–6.10 g/cm³, making it one of the densest ceramic materials. Structurally, zirconia exists in three crystallographic phases—monoclinic, tetragonal, and cubic—each with distinct properties. The tetragonal and cubic zirconia are particularly desirable in engineering applications due to their higher toughness and stability, often stabilized by doping with yttria (Y₂O₃).
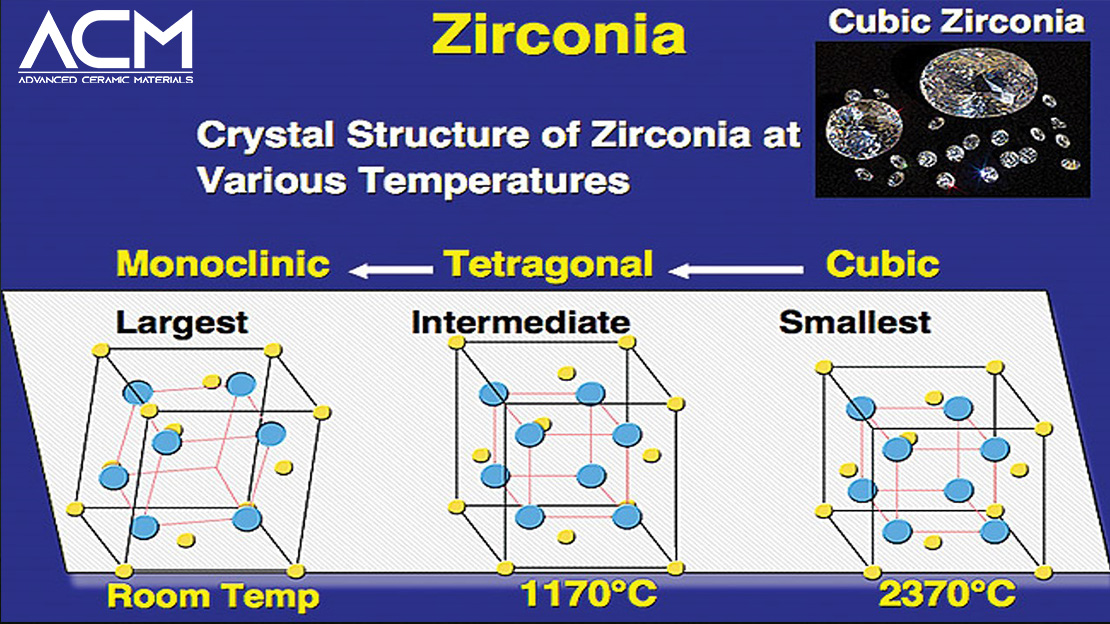
Zirconia's extreme hardness and insolubility in water and most acids contribute to its resilience in demanding settings, including chemical plants and precision tools.
Physical Characteristics Table:
| Property | Description/Value |
|---|---|
| Appearance | White to yellowish powder or solid |
| Odor | No odor |
| Specific Density | 5.68–6.10 g/cm³ |
| Crystal Phases | Monoclinic, tetragonal, cubic |
| Stabilizer Additives | Yttria, magnesia, calcia |
| Solubility | Insoluble in water and most acids |
These features ensure zirconia maintains structural integrity and performance under extreme pressures and corrosive environments, making it ideal for medical, industrial, and electronics applications.
Mechanical Properties of Zirconia
Zirconia is best known for its exceptional mechanical strength and fracture toughness. Yttria-stabilized zirconia (YSZ), for instance, exhibits a fracture toughness of up to 10 MPa·m^0.5, among the highest of all ceramics. This toughness stems from a transformation toughening mechanism, where stress-induced phase changes absorb energy and impede crack growth.
Its flexural strength can reach 1000 MPa, while the Vickers hardness ranges from 11–13 GPa. This balance of strength and resistance to wear makes zirconia ideal for cutting instruments, valve seats, and structural supports.
Mechanical Properties Table:
| Property | Value |
|---|---|
| Fracture Toughness | 7–10 MPa·m^0.5 |
| Flexural Strength | Up to 1000 MPa |
| Hardness (Vickers) | 11–13 GPa |
| Elastic Modulus | ~200 GPa |
Zirconia’s combination of hardness and toughness places it at the forefront of ceramic materials used in mechanically demanding environments.
Thermal and Electrical Properties
Zirconia offers impressive thermal stability, with a melting point around 2700°C. While its thermal conductivity is relatively low (about 2–3 W/m·K), this feature becomes advantageous in thermal barrier coatings, especially in aerospace and turbine applications.
YSZ is also a solid electrolyte at high temperatures, conducting oxygen ions efficiently—making it crucial in fuel cells and oxygen sensors. Its low thermal expansion coefficient (around 10 x10⁻⁶/°C) enhances its compatibility with metals and other ceramics in multilayer assemblies.
Thermal and Electrical Properties Table:
| Property | Value |
|---|---|
| Melting Point | ~2700°C |
| Thermal Conductivity | 2–3 W/m·K |
| Coefficient of Expansion | ~10 x10⁻⁶/°C |
| Ionic Conductivity (YSZ) | High at elevated temperatures |
| Electrical Conductivity | Insulator at room temperature |
These properties support the widespread use of zirconia in energy, aerospace, and electronic applications where high-temperature endurance and electrical insulation are essential.
Chemical Properties and Resistance
Zirconia is chemically inert across a wide range of acidic and basic conditions. It does not easily degrade in corrosive environments, making it an excellent material for chemical contact parts. The stability of zirconia powder and its final sintered forms ensures long-term performance in harsh chemical processes.
Additionally, zirconia’s oxidation resistance up to 1000°C (and even higher when stabilized) ensures its reliability in oxidizing atmospheres.
Chemical Properties and Resistance Table:
| Property | Description |
|---|---|
| Chemical Stability | High, resistant to acids, alkalis, salts |
| Oxidation Resistance | Excellent, even at elevated temperatures |
| Thermal Shock Resistance | Strong due to transformation toughening |
| Corrosion Resistance | High, suitable for aggressive environments |
Zirconia’s chemical inertness enhances its reliability in hostile settings, contributing to longer service life and fewer maintenance cycles in industrial systems.
Conclusion
Zirconia stands out as a ceramic material that uniquely balances strength, toughness, chemical stability, and thermal resistance. Its versatility enables its use in a wide array of high-performance applications—from dental crowns to turbine blades to solid oxide fuel cells.
Suppliers like Advanced Ceramic Materials (ACM) offer a full range of zirconia products, including powders, rods, tubes, and custom components tailored to your specific needs. Whether you're in search of structural ceramics or high-temperature insulators, zirconia offers the right mix of durability and precision.
As technology advances and demands for robust, adaptable materials continue to grow, zirconia is well-positioned to play a vital role in shaping the future of advanced manufacturing and clean energy.
Further Reading
-
Different Compositions of Zirconia and Their Applications: Explore the different types of zirconia ceramics and their uses in industry and medicine. Read more.
-
How Yttria-Stabilized Zirconia Works: Learn about the science behind phase-stabilized zirconia and why it's critical in high-stress applications. Read more.
-
Top Applications of Zirconia in Modern Industry: A guide to the practical applications of zirconia ceramics across multiple sectors. Read more.
{{item.content}}
LEVE A REPLY
{{item.children[0].content}}
{{item.content}}
LEAVE A REPLY
SUBSCRIBE OUR NEWSLETTER
- How PBN Crucibles Ensure the Quality of GaN & SiC Epitaxial Materials
- SiC vs. Quartz Focus Rings: A Cost and Performance Analysis for Advanced Etch
- AlN Ceramic Substrates: Enabling Next-Gen Electrostatic Chucks
- The Amor of Semiconductor Tools: Why High-Purity Al2O3 & AlN Are Preferred for Plasma Process Chambers
- Silicon Carbide - Ultra-High Temperature Ceramics for Extreme Environments