Boron Nitride Overview: Properties, Production, and Uses
Overview
Boron nitride is a non-toxic thermal and chemical refractory compound with high electrical resistance and low density, commonly found in colorless crystals or white powder. As an advanced ceramic material, boron nitride has a unique structure that gives it properties similar to both graphite and diamond, earning it nicknames like "white graphene" or "inorganic graphite." With its diverse applications and remarkable physical properties, boron nitride is widely studied and used in industries ranging from electronics to cosmetics. In this article, we will explore its properties, density, structure, production methods, and uses.
Properties of Boron Nitride
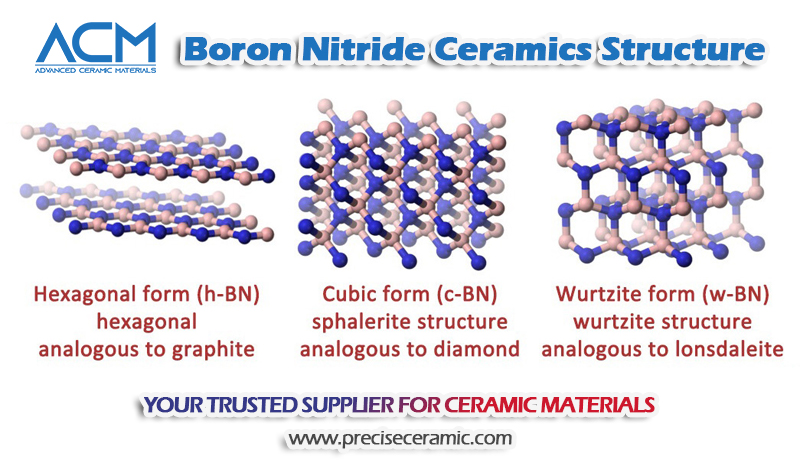
The structure of boron nitride consists of equal numbers of boron and nitrogen atoms, forming a robust lattice that gives rise to its unique physical and chemical properties. Depending on how the atoms are arranged, boron nitride exists in three main crystalline forms:
- Hexagonal Boron Nitride (h-BN): A layered, graphite-like structure known for its lubricating and insulating properties.
- Cubic Boron Nitride (c-BN): A diamond-like structure with exceptional hardness and oxidation resistance.
- Wurtzite Boron Nitride (w-BN): A rarer form, considered even harder than cubic boron nitride under certain conditions.
These structural variations are responsible for boron nitride’s wide range of applications, as each form offers distinct thermal, mechanical, and chemical characteristics.
| Property | Hexagonal BN (h-BN) | Cubic BN (c-BN) | Wurtzite BN (w-BN) |
|---|---|---|---|
| Structure | Graphite-like, layered | Diamond-like, cubic | Similar to c-BN, hexagonal |
| Hardness | Low | Very high (2nd to diamond) | Higher than diamond |
| Density (g/cm³) | ~2.1 | ~3.48 | Similar to c-BN |
| Thermal Conductivity | High | High | High |
| Applications | Lubricants, insulators | Cutting tools, abrasives | Advanced manufacturing |
Other key properties of boron nitride include:
- High Thermal Conductivity: Essential for heat dissipation in electronics and high-temperature environments.
- Chemical Inertness: Makes it resistant to corrosion by acids, alkalis, and molten metals.
- Low Density: h-BN has a density of ~2.1 g/cm³, while c-BN is denser at ~3.48 g/cm³.
- Electrical Insulation: Ensures reliable performance as a dielectric material.
- High Melting Point: Withstands temperatures up to 2,973°C, making it suitable for extreme conditions.
Production of Boron Nitride
Boron nitride is typically synthesized through chemical reactions between boric acid or boron oxide and nitrogen under controlled conditions. The production methods include:
Hexagonal Boron Nitride (h-BN):
- Produced by reacting boric acid with ammonia in a nitrogen atmosphere.
- Dense shapes are formed through hot pressing due to its poor sinterability.
Cubic Boron Nitride (c-BN):
- Created by subjecting hexagonal boron nitride to high pressure and temperature, mimicking the process used to produce synthetic diamonds.
Wurtzite Boron Nitride (w-BN):
- Formed under slightly different conditions compared to c-BN, specifically at lower temperatures (~1,700°C).
Boron nitride can be manufactured in various forms, including powders, bars, rods, and plates. The material’s density and grade (e.g., A, AX, 05, HP, M) vary depending on its intended application, ensuring adaptability across industries.
Uses Of Boron Nitride
Boron nitride's unique structure and density enable it to serve a wide range of applications across multiple industries. Its versatility stems from its various crystalline forms, including hexagonal boron nitride (h-BN), cubic boron nitride (c-BN), and wurtzite boron nitride (w-BN). These forms collectively contribute to its exceptional performance in challenging environments. Below are the key applications of boron nitride.
Industrial and Manufacturing:
Boron nitride is widely used in cutting and grinding tools for hard materials such as hardened steel and wear-resistant cast iron, thanks to its high hardness and chemical stability.
Its thermal conductivity and resistance to molten metals make it a preferred material in high-temperature furnaces, vacuum systems, and thermal spraying applications.
Electronics and Optics:
The material's low dielectric constant, excellent thermal stability, and electrical insulation properties make it suitable for use in semiconductor heat sinks and as a substrate material for graphene-based devices.
In the optics industry, boron nitride's ability to resist oxidation and its high thermal conductivity enable its application in advanced optical coatings and electronics.
Automotive and Aerospace:
Hexagonal boron nitride is commonly used for creating seals and insulating components in the automotive industry, such as oxygen sensors and thermal shields.
Its lightweight density and structure contribute to its use in aerospace materials where weight reduction and thermal resistance are critical.
Cosmetics and Medical:
Boron nitride’s lubricious nature and non-toxicity make it ideal for cosmetics, including eye shadows, foundations, and lipsticks, where it improves smoothness and spreadability.
Emerging research suggests potential applications in the biomedical field, such as implants and biocompatible coatings.
Other Applications:
Boron nitride is frequently used in the production of coatings for tools and molds to enhance their wear resistance.
It also finds applications in ceramics, paints, resins, and high-performance alloys.
Final Thoughts
Boron nitride stands out as a highly versatile material with exceptional structure and density that support its wide range of industrial and scientific applications. Its properties, including high thermal conductivity, chemical inertness, and electrical insulation, make it indispensable in industries such as electronics, aerospace, cosmetics, and manufacturing.
With advancements in production techniques, boron nitride is becoming more accessible and finding new uses in cutting-edge technologies like graphene-based devices, optical electronics, and nanotechnology. Its potential in emerging fields, such as biomedicine and energy storage, suggests that the full scope of boron nitride’s applications is yet to be realized.
Despite being classified as non-toxic, precautions should be taken during processes like grinding or cutting, which may release airborne particulates. As research continues to uncover more about its properties and uses, boron nitride is set to play a crucial role in the future of materials science and technology.
{{item.content}}
LEVE A REPLY
{{item.children[0].content}}
{{item.content}}